[1] J. M. Gregio, M. A. L. Caetano and T. Yoneyama, State estimation
and optimal long period clinical treatment of HIV seropositive
patients, Anais da Academic Brasileira de Ciencias 81(1) (2009),
3-12.
DOI: http://dx.doi.org/10.1590/S0001-37652009000100002
[2] B. E. Bassey and Lebedev K. Andreyevich, On analysis of parameter
estimation model for the treatment of pathogen-induced HIV
infectivity, Open Access Library Journal 3(4) (2016), 1-13.
DOI: http://dx.doi.org/10.4236/oalib.1102603
[3] R. V. Culshaw, S. Ruan and R. J. Spiteri, Optimal HIV treatment by
maximizing immune response, Journal of Mathematical Biology 48(5)
(2004), 545-562.
DOI: https://doi.org/10.1007/s00285-003-0245-3
[4] H. Zhu and X. Zou, Dynamics of a HIV-1 infection model with
cell-mediated immune response and intracellular delay, Discrete and
Continuous Dynamical Systems, Series B 12(2) (2009), 511-524.
DOI: https://doi.org/10.3934/dcdsb.2009.12.511
[5] D. Kirschner, Dynamics of co-infection with M. tuberculosis and
HIV-1, Theoretical Population Biology 55(1) (1999), 94-109.
DOI: https://doi.org/10.1006/tpbi.1998.1382
[6] B. E. Bassey and K. A. Lebedev, On mathematical modeling of the
effect of bi-therapeutic treatment of tuberculosis epidemic, Journal
of Modern Mathematics and Statistics 9(1-4) (2015), 1-7.
[7] E. B. Bassey, R. A. Kimbir and K. A. Lebedev, On optimal control
model for the treatment of dual HIV-parasitoid pathogen infection,
Journal of Bioengineer & Biomedical Science 7(1) (2016), 1-7.
DOI: https://doi.org/10.4172/2155-9538.1000212
[8] R. A. Arnaout, M. A. Nowak and D. Wodarz, HIV-1 dynamics
revisited: Biphasic decay by cytotoxic T lymphocyte killing?, Proc.
Roy. Soc. Lond. B 267(1450) (2000), 1347-1354.
DOI: https://doi.org/10.1098/rspb.2000.1149
[9] K. Hattaf and N. Yousfi, Optimal control of a delayed HIV
infection model with immune response using an efficient numerical
method, ISRN Biomathematics 2012, pages 7, Article ID 215124.
DOI: http://dx.doi.org/10.5402/2012/215124
[10] M. A. Nowak and C. R. Bangham, Population dynamics of immune
responses to persistent viruses, Science 272(5258) (1996), 74-79.
DOI: http://dx.doi.org/10.1126/science.272.5258.74
[11] J. E. Schmitz et al., Control of Viremia in simian
immunodeficiency virus infection by 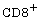 lymphocytes, Science 283(5403) (1999),
857-860.
lymphocytes, Science 283(5403) (1999),
857-860.
DOI: http://dx.doi.org/10.1126/science.283.5403.857
[12] X. Jin et al., Dramatic rise in plasma Viremia after 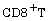 cell depletion in simian immunodeficiency
virus-infected macaques, Journal of Experimental Medicine 189(6)
(1999), 991-998.
cell depletion in simian immunodeficiency
virus-infected macaques, Journal of Experimental Medicine 189(6)
(1999), 991-998.
DOI: http://dx.doi.org/10.1084/jem.189.6.991
[13] E. S. Rosenberg, M. Altfeld, S. H. Poon, M. N. Phillips, B. M.
Wilkes, R. L. Eldridge, G. K. Robbins, R. T. D’Aquila, P. J.
Goulder and B. D. Walker, Immune control of HIV-1 after early
treatment of acute infection, Nature 407 (2000), 523-526.
DOI: http://dx.doi.org/10.1038/35035103
[14] D. Wodarz, K. M. Page, R. A. Arnaout, A. R. Thomsen, J. D. Lifson
and M. A. Nowak, A new theory of cytotoxic T-lymphocyte memory:
Implications for HIV treatment, Philos. Trans. R. Soc. Lond. B: Biol.
Sci. 355(1395) (2000), 329-343.
DOI: http://dx.doi.org/10.1098/rstb.2000.0570
[15] D. Wodarz, R. M. May and M. A. Nowak, The role of
antigen-independent persistence of memory cytotoxic T lymphocytes,
International Immunology 12(4) (2000), 467-477.
DOI: https://doi.org/10.1093/intimm/12.4.467
[16] A. R. Thomsen, J. Johansen, O. Marker and J. P. Christensen,
Exhaustion of CTL memory and recrudescence of viremia in lymphocytic
choriomeningitis virus-infected MHC class II-deficient mice and B
cell-deficient mice, Journal of Immunology 157(7) (1996),
3074-3080.
[17] A. R. Thomsen, A. Nansen, J. P. Christensen, S. O. Andreasen and
O. Marker, CD40 Ligand is pivotal to efficient control of virus
replication in mice infected with lymphocytic choriomeningitis virus,
Journal of Immunology 161(9) (1998), 4583-4590.
[18] P. Borrow, A. Tishon, S. Lee, J. Xu, I. S. Grewal, M. B. Oldstone
and R. A. Flavell, CD40L-Deficient mice show deficits in antiviral
immunity and have an impaired memory 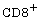 CTL response, Journal of Experimental Medicine
183(5) (1996), 2129-2142.
CTL response, Journal of Experimental Medicine
183(5) (1996), 2129-2142.
DOI: http://dx.doi.org/10.1084/jem.183.5.2129
[19] P. Borrow, D. F. Tough, D. Eto, A. Tishon, I. S. Grewal, J.
Sprent, R. A. Flavell and M. B. A. Oldstone, CD40 Ligand-mediated
interactions are involved in the generation of memory 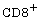 cytotoxic T lymphocytes (CTL) but are not
required for the maintenance of CTL memory following virus infection,
Journal of Virology 72(9) (1998), 7440-7449.
cytotoxic T lymphocytes (CTL) but are not
required for the maintenance of CTL memory following virus infection,
Journal of Virology 72(9) (1998), 7440-7449.
[20] D. Wodarz and M. A. Nowak, Specific therapy regimes could lead to
long-term immunological control of HIV, Proc. Natl. Acad. Sci. USA
96(25) (1999), 14464-14469.
[21] H. Zarei, A. V. Kamyad and S. Effati, Maximizing of asymptomatic
stage of fast progressive HIV infected patient using embedding method,
Intelligent Control and Automation 1(1) (2010), 48-58.
DOI: http://dx.doi.org/10.4236/ica.2010.11006
[22] A. Landi, A. Mazzoldi, C. Andreoni, M. Bianchi, A. Cavallini, M.
Laurino, L. Ricotti, R. Iuliano, B. Matteoli and L. Ceccherini-Nelli,
Modelling and control of HIV dynamics, Computer Methods and Programs
in Biomedicine 89(2) (2008), 162-168.
DOI: https://doi.org/10.1016/j.cmpb.2007.08.003
[23] Bassey E. Bassey, Optimal control model for immune effectors
response and multiple chemotherapy treatment (MCT) of dual delayed HIV
pathogen infections, SDRP Journal of Infectious Diseases Treatment &
Therapy 1(1) (2017), 1-18.
[24] B. E. Bassey, Quantum optimal control dynamics for delay
intracellular and multiple chemotherapy treatment (MCT) of dual
delayed HIV-pathogen infections, International Journal of Scientific
and Innovative Mathematical Research 5(6) (2017), 1-19.
http://dx.doi.org/10.20431/2347-3142.0506001
[25] B. E. Bassey, Dynamic optimal control model for period multiple
chemotherapy (PMC) treatment of dual HIV-pathogen infections, Journal
of Analytical & Pharmaceutical Research 6(3) 00176 (2017), 1-22.
DOI: http://dx.doi.org/10.15406/japlr.2017.06.00176
[26] D. Wodarz and M. A. Nowak, Mathematical models of HIV
pathogenesis and treatment, BioEssays 24(12) (2002), 1178-1187.
DOI: https://doi.org/10.1002/bies.10196
[27] J. Hale and S. M. Verduyn Lunel, Introduction to Functional
Differential Equations, Applied Mathematical Science 99,
Springer-Verlag, New York, 1993.
[28] A. S. Perelson and P. W. Nelson, Mathematical analysis of HIV-1
dynamics in vivo, SIAM Review 41(1) (1999), 3-44.
DOI: https://doi.org/10.1137/S0036144598335107
[29] S. Butler, D. Kirschner and S. Lenhart, Optimal control of
chemotherapy affecting the infectivity of HIV, Editors: O. Arino, D.
Axelrod and M. Kimmel, Advances in Mathematical Population
Dynamics-Molecules Cells and Man (1997), 557-569.
[30] W. H. Fleming and R. W. Rishel, Deterministic and Stochastic
Optimal Control, Springer Verlag, New York, 1975.
[31] S. A. Perelson, E. D. Kirschner and R. De Boer, Dynamics of HIV
infection of  T cells, Mathematical Biosciences 114(1)
(1993), 81-125.
T cells, Mathematical Biosciences 114(1)
(1993), 81-125.
DOI: https://doi.org/10.1016/0025-5564(93)90043-A
[32] D. L. Lukes, Differential Equations: Classical to Controlled,
Vol. 162 of Mathematics in Science and Engineering, Academic Press,
New York, NY, USA, 1982.
[33] H. R. Joshi, Optimal control of an HIV immunology model, Optimal
Control Applications and Methods 23(4) (2002), 199-213.
DOI: https://doi.org/10.1002/oca.710
[34] K. R. Fister, S. Lenhart and J. S. McNally, Optimizing
chemotherapy in an HIV model, Electronic Journal of Differential
Equations 32 (1998), 1-12.
[35] X. Jin et al., An antigenic threshold for maintaining human
Immunodeficiency virus type 1-specific cytotoxic T lymphocytes,
Molecular Medicine 6(9) (2000), 803-809.