Author's: Feng-Yun Wang, Guo-Bin Jung, Shi-Jun Luo, Chang-Ling Wang, Xiao Hao, Chi-Yuan Lee and Feng-Bor Weng
Pages: [81] - [96]
Received Date: November 27, 2009
Submitted by:
A new method for the preparation of nano-ceria of high thermal
stability was systematically studied by using XRD, TEM, ICP-AES,
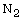 physical adsorption and desorption, and an
equipment combining the functions of thermal gravimetry, differential
scanning calorimeter, and mass spectrometer. The method includes the
synthesis of
physical adsorption and desorption, and an
equipment combining the functions of thermal gravimetry, differential
scanning calorimeter, and mass spectrometer. The method includes the
synthesis of 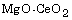 mixed powder by calcining a gel made with
cerium nitrate, magnesium nitrate, and citric acid, and the removing
of MgO from the mixed powder by acetic acid leaching. The calcining
process mainly included two weight-loss steps. Step 1 took place at a
lower temperature, which decreasing from 535K to 480K slightly with
Mg/Ce increasing from 1 to 14, probably due to the reaction between
the organic fragments and nitrogen oxides from the nitrates, and the
catalytic function of MgO. Step 2 took place at a higher temperature,
which increasing from 620K to 740K apparently with Mg/Ce increasing,
due to the reaction between the organic fragments and
mixed powder by calcining a gel made with
cerium nitrate, magnesium nitrate, and citric acid, and the removing
of MgO from the mixed powder by acetic acid leaching. The calcining
process mainly included two weight-loss steps. Step 1 took place at a
lower temperature, which decreasing from 535K to 480K slightly with
Mg/Ce increasing from 1 to 14, probably due to the reaction between
the organic fragments and nitrogen oxides from the nitrates, and the
catalytic function of MgO. Step 2 took place at a higher temperature,
which increasing from 620K to 740K apparently with Mg/Ce increasing,
due to the reaction between the organic fragments and 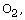 and the catalytic function of
and the catalytic function of 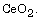 The MgO in the mixed powder could be
completely removed by reaction with acetic acid solution (2.5 vol.%)
at room temperature, but the
The MgO in the mixed powder could be
completely removed by reaction with acetic acid solution (2.5 vol.%)
at room temperature, but the  remained unaffected. In the calcining
process, the freshly formed ceria particles could thermally be
stabilized in the rest calcining, but did not agglomerate because the
formed MgO particles might act as isolators. Consequently, the
resulting ceria powders showed high surface area and high thermal
stability.
remained unaffected. In the calcining
process, the freshly formed ceria particles could thermally be
stabilized in the rest calcining, but did not agglomerate because the
formed MgO particles might act as isolators. Consequently, the
resulting ceria powders showed high surface area and high thermal
stability.
ceria preparation, MgO, thermal stability, new method.